 failures.Of course,one could use teflon coated tubes as in condensing exchangers,but the cost may be significant. A simple solution is to ensure that the lowest tube wall or surface
temperature is above the acid dew point. One of the suggestions engineers
offer is to increase flue gas temperature at the air heater exit. This
solution may work in the case of air heater,where the heat transfer coefficients
on the flue gas and air side are nearly equal and hence increasing the
flue gas temperature increases the average back end temperature. However
in the case of an economizer this does not work,as shown by the following
calculations.
failures.Of course,one could use teflon coated tubes as in condensing exchangers,but the cost may be significant. A simple solution is to ensure that the lowest tube wall or surface
temperature is above the acid dew point. One of the suggestions engineers
offer is to increase flue gas temperature at the air heater exit. This
solution may work in the case of air heater,where the heat transfer coefficients
on the flue gas and air side are nearly equal and hence increasing the
flue gas temperature increases the average back end temperature. However
in the case of an economizer this does not work,as shown by the following
calculations.
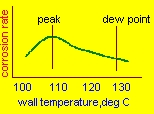
| Computing tube wall temperatures The average wall temperature of a bare tube economizer is given by the simple equation,neglecting small resistances such as due to fouling: tw=tg-(tg-ti)xhi/(hi+ho) tw=average tube wall temperature tg,ti= gas and water temperatures hi,ho= tube side and gas side heat transfer coefficients In an economizer,hi is about 1000 and ho=15 Btu/ft2hF Let us look at two cases: 1.gas temperature=750 F and water temperature=250 F tw=750-(750-250)x1000/1015 =258 F 2.gas temperature=350 F tw=350-(350-250)x1000/1015 = 252 F Hence it is obvious that increasing the gas temperature to avoid corrosion will not work as the tube side coefficient is so high compared to the gas side that the tube wall temperature will be close to the water temperature! Hence the right solution is to increase the feed water temperature using a pre-heater. How much should the temperature be raised?Lower the feed water temperature,lower the boiler exit gas temperature and hence higher the efficiency. A 40 F drop in gas temperature results in about 1 % improvement. The figure above shows the corrosion rate versus tube wall temperature.Typically the maximum corrosion rate does not occur at the dew point but about 15-20 deg C lower. This means that a little more energy can be recovered from the flue gases if we raise the feed water to about 10-15 C of the dew point.Of course to be absolutely sure,we can increase the feed water to above the dew point. Concocting ideas such as going for parallel flow instead of counter flow in the economizer arrangement or increasing the gas exit temperature to 500 F etc will not help as seen in the above calculation.(Unfortunately I have seen thousands of specifications on boilers where consultants suggest these ideas if corrosion is likely!) |
Dew
Point of Acid Vapors
Hydrochloric acid 1000/Tdp =3.7368-0.1591 ln(Ph2o)-.0326 ln(Phcl)+0.00269 ln(Ph2o)ln(Phcl) Sulfuric acid 1000/Tdp=2.276-0.0294 ln(Ph2o)-.0858 ln(Ph2so4)+0.0062 ln(Ph2o)ln(Ph2so4) The dew points are in deg K and partial pressures in mm Hg. If a flue gas contains 12 % vol of water vapor and 0.02 % vol SO2 and say 2 % of SO2 converts to SO3,compute the sulfuric acid dew point. Gas pressure=10 in wg or (10/407) =0.02457 atmg or 1.02457 ata. Ph2o =0.12x1.02457x760=93.44 mm Hg. ln(Ph2o)=4.537 Pso3 is equal to Ph2so4. Pso3=0.02x.0002x(64/80)x760x1.02457=0.0024917 ln(Pso3)= -6. Note that 2 % conversion is on weight basis and hence we multipled and divided by the molecular weights of so2 and so3 in the above calculation. 1000/Tdp = 2.276-0.0294x4.537+0.0858x6-0.0062x4.537x6=2.489 or Tdp=402 K or 129 C or 264 F |
